It has 21 electrons and 19 protons d. A Mass number of the atom.

Solved Ion Has 12 Protons 14 Neutrons And 10 Electrons Chegg Com
28 Protons14 Neutrons14 Electrons10 To find the neutrons do the mass number of the element minus the atomic number of the element.

. We need to find the charge and the number of protons and electrons after formation of ions. X Symbol of the atom. Protons and Neutrons in Magnesium.
Answer 1 of 5. It has 19 electrons and 20 neutrons b. April 21 2022.
Therefore net charge 12-10 2 2 units of positive charge. Number of neutrons 14. Sports drink e black coffee Which of the following is an example of the law of multiple proportions.
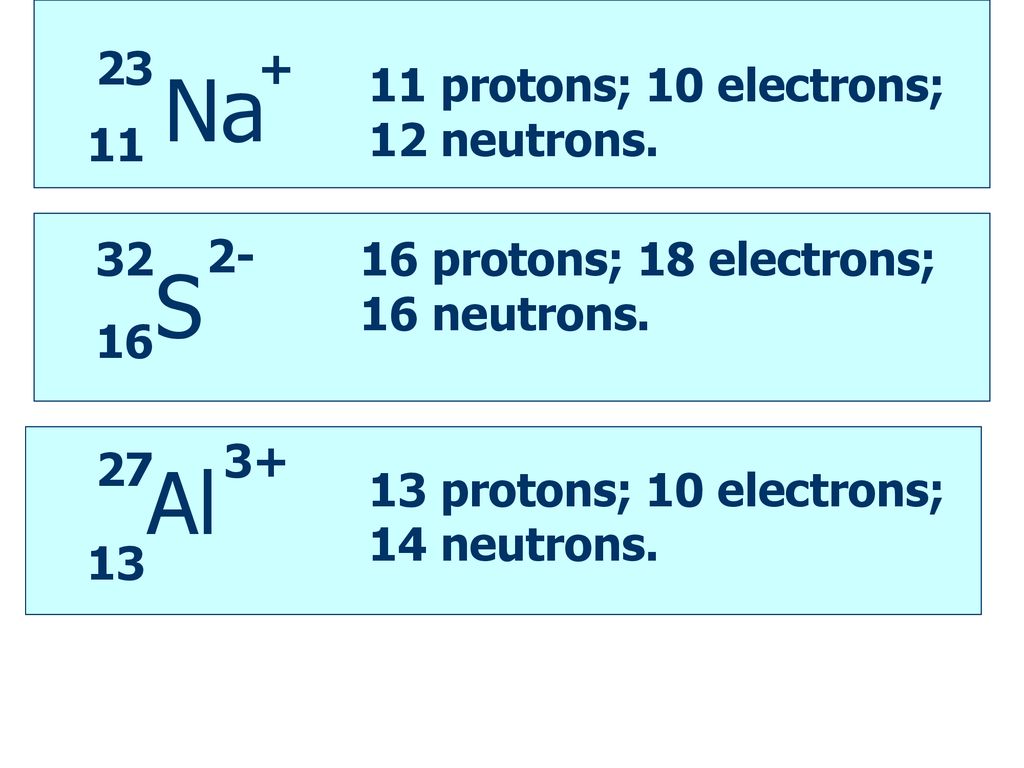
26 Mg 2 e. 26 Mg 2 e. 19 An ion has 12 protons 13 neutrons and 10 electrons.
American college of gastroenterology gerd guidelines. The symbol for the ion is 19 A 25Ne2- D 23AI E25Mg2 20 What period 3 element has the following ionization energies all in kJmol. The plus tells us that electrons have been lost and that 2 tells us how many electrons have been lost.
Hence the ion will have. Boron 10 protons neutrons electrons. Ion has 12 protons 14 neutrons and 10 electrons.
Since it has 12 protons that means its. Where Z Atomic number of the atom. Uncategorized boron 10 protons neutrons electrons.
The symbol for the ion is a. Number of protons 12. It has 19 protons and 19 neutrons c.
Magnesium2 because it donated 2 electrons. Following statements is TRUE about the atom. When the atoms loses 2 electrons then the atom comprises of 10 electrons.
A a negatively charged nucleus surrounded by positively charged protons. Chemistry questions and answers. Itloses 2 electrons to form an ion.
Hence we can conclude that there are 7 electrons 7 protons and 7 neutrons that nitrogen-14 have and it isnt an anion or cation. Since a stable atom has a net charge of 0 we must have 20 electrons. Magnesium is a nutrient required by the body to remain healthy.
Carbon -12 and carbon -13 are both stable while carbon - 14 is unstable and has a half-life of 5730 40 years. 1What is the overall charge of an ion that has 12 protons 10 electrons and 14 neutrons. Mg and has an atomic number of 12 which means 12 protons are in it.
Total number of protons in the nucleus is called the atomic number of the atom and is given the symbol ZThe total electrical charge of the nucleus is therefore Ze where e elementary charge equals to 1602 x 10-19 coulombs. Legends of runeterra viego is broken boron 10 protons neutrons electrons boron 10 protons neutrons electrons. 26 Ne 2- d.
24 Si 2 Choose the heterogeneous mixture from the list below a. Magnesium Ion has 12 protons and 10 neutrons. Chlorine gas c carbon graphite d.
How Many Protons And Electrons Does Calcium Have20 protonsHow many protons electrons and neutrons does calcium haveCalcium is the 20th element with 20 protons since the number of protons directly changes the element itself. It is a neutral atom. Chemistry questions and answers.
Since it has 12 protons that means its atomic number is 12 and we know the element with atomic number 12 is Magnesium Mg. 26 Ne 2- d. 24 Si 2 Choose the heterogeneous mixture from the list below a.
The symbol for the ion is a. Atomic mass Number of protons Number of neutrons. The atom of an element is described using the following data.
If it has 12 protons that means it has 12 units of positive charge and 10 electrons which means 10 units of negative charge. Given that an atom X has 12protons and 12electrons. The isotopic representation of an atom is.
What element has 12 protons 14 neutrons and 10 electrons. By Apr 17 2022 rent ps4 console gamestop construction and demolition waste management pdf. 112 rows Oxygen has 8 protons 8 neutrons and 8 electrons.
Californium protons neutrons electronsvans for sale under 4000. A an electron and an alpha particle B an electron and a proton C a neutron and an alpha particle D a neutron and a proton 2Which particles have approximately the same mass. If it has 12 protons that means it has 12 units of positive charge and 10 electrons which means 10 units of negative charge.
Carbon-14 14 C or radiocarbon is a radioactive isotope of carbon with an atomic nucleus containing 6 protons and 8 neutrons. Therefore net charge 12 -10 2 2 units of positive charge. Ion has 12 protons 14 neutrons and 10 electrons.
Fluorine has 9 protons 10. What will be the number of protons and electrons in the ion. We should note that the total number of neutrons present in the nucleus is equal to the difference between the atomic number and the mass number of an atom.
Magnesium is a chemical element with atomic number 12 which means there are 12 protons in its nucleus. Number of electrons 10. Were looking at the charge next which is 2.
Boron 10 protons neutrons electrons. Posted on April 18 2022 by. It is a neutral atom.
Atomic Mass 12 14 26.
Subatomic Particles In Ions Ppt Download

0 Comments